TÜV CERTIFIED* AND HEALTH CANADA-CLEARED MEDICAL DEVICE
Weight 3.4 kg (7.5 lbs)
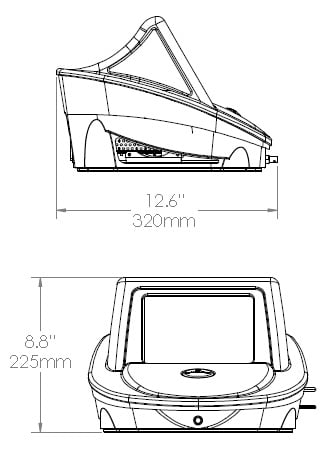
Width 33 cm (13’’)
Height 23 cm (9.1’’)
Depth 31.5 cm (12.4’’)
Voltage 120 / 230 V
Frequency 60 / 50 Hz
Current 1.0 / 0.5 A
Protection Class 1 (Grounded)
Patient Applied Part Type BF
Operating Mode Continuous
Maximum Output Power 10 W
Tolerance/Accuracy of Display 15%
Rated Load 1 KOhm
Rated Output Frequency 4 MHz
Maximum Output Voltage 300 VAC
Materials: Steel & Thermoform casting
Electronics Microprocessor controlled
(*) Tested to comply with the applicable standards and equipment specifications:
ICES-003:2004.
FCC Part 15 Subpart B:2010 & EN 60601-1-2:2007-(Ed 3.0) & EN 60601-2-2.
CAN/CSA 22.2 No. 60601-1:2008 excluding Biocompatibility (clause 11.7), Usability (clauses 7.1.1 and 12.2), PEMS (clause 14), and EMC (clauses 7.9.2.2 and 17).
CAN/CSA C22.2 No. 60601-2-2:2009 excluding EMC (clause 17).
UL 60601-1:2003 excluding EMC (clauses 6.8.2 and 36), Biocompatibility (clause 48), and PEMS (clause 52.1 as it pertains to IEC 60601-1-4).
IEC 60601-2-2:2006 excluding EMC (clause 36).
ANSl/AAMI ES 60601-1/A2:2010 excluding Biocompatibility (clause 11.7), Usability (clauses 7.1.1and12.2), PEMS (clause 14), and EMC (clauses 7.9.2.2 and 17).
IEC 60601-2-2:2009 excluding EMC (clause 17).